Stellar Nucleosynthesis
Element Origins
After the Big Bang, the ordinary matter in the Universe was composed primarily of just two elements: hydrogen and helium. Currently, there are a wide range of elements, from argon to zirconium. These more complex elements were nearly all produced via stellar evolution processes. The formation of new elements is called nucleosynthesis. Below, I have describe the basics of the chemistry of the Universe and how it has changed over time.
Big Bang Nucleosynthesis
The
star-forming region 30 Doradus. The blue stars are massive, hot, young
stars. They have formed in a cluster out of gas and dust. (Credit: NASA,
ESA, HST WFC3)
When the Big Bang occurred approximately 14 billion years ago, the Universe was very hot and dense. Over time, the Universe expanded and cooled down. Approximately 0.001 seconds after the Big Bang, particles (e.g., protons and neutrons) began to fuse together to form atomic nuclei, dubbed nuclear fusion. Nuclear fusion processes depend on the density and temperature of the environment. More complex atomic nuclei require dense, hot environments; particles are more likely to smack into each other and stick together in these conditions. Although the Big Bang gave rise to an incredibly dense and hot environment, it was also expanding at a great rate. Approximately 5 minutes after the Big Bang, the Universe was no longer hot and dense enough to continue fusion. At this point, 75% of the ordinary matter in the universe (by mass) was Hydrogen nuclei. 25% had combined into helium nuclei. There were also trace amounts of other elements, namely deuterium (a hydrogen atom with an additional neutron) and lithium. This process is dubbed Big Bang Nucleosynthesis (BBN).
The chemical make up of the Universe is still representative of BBN. However, it also has a small fraction of more complex elements, which astronomers refer to as metals or heavy elements, produced via stars.
Stellar Nucleosynthesis
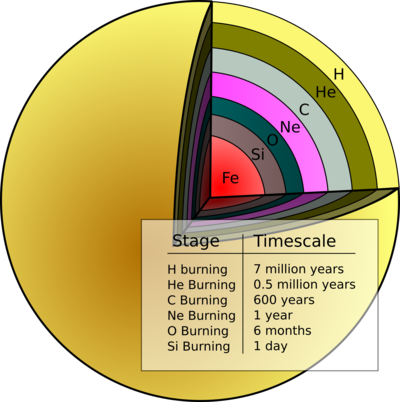
The next generation of stars formed out of material that had been enriched by the nuclear fusion products of the first. Just like the first generation, these stars initially burn hydrogen in their cores, fusing it into helium atoms. After a period of time, the star has processed much of the core hydrogen and this fusion process no longer releases enough energy to counteract the inward pull of gravity. The stellar core contracts, reaching temperatures and densities that support nuclear fusion of helium into carbon. Hydrogen fusion persists in less dense portions of the star. For more massive stars, this pattern continues as the star creates more elements, such as carbon, neon, oxygen, silicon, and iron. Whereas for elements less massive than iron, nuclear fusion released energy, beyond this element fusion requires an energy source. Once the material in a stellar core has fused to iron, it can no longer produce energy and support itself from gravitational collapse. The star will collapse and explode as a supernova, releasing all of the elements it produced through fusion into its surroundings. There are additional nucleosynthetic processes that occur in supernovae and, via neutron-capture, form elements heavier than iron, such as europium.
Low-mass stars, like the Sun, have a simpler life than those with high mass. The lowest mass stars are unable to reach appropriate temperatures to fuse helium, ending their lives as white dwarfs. For those that can, core nuclear fusion cannot move beyond carbon. It takes very large temperatures, which low mass stars are unable to reach, to fuse carbon. Without pressure support, the core gravitationally shrinks. This increases the temperature and density of material that surrounds the core, igniting hydrogen and helium fusion in layers. Fusion in these shells occurs at a much more rapid rate than in the core, producing large amounts of energy. This results in the shells expanding outwards, as the pressure support overcomes the gravitational pull. Astronomers suspect that there are additional nucleosynthetic processes that occur at this life stage; through neutron-capture, the stellar material combines to form elements beyond iron. Stellar winds then remove material from these puffed-up outer layers, releasing the enriched material into the surrounding environment. Eventually, these outer layers are ejected into space from the carbon stellar core, creating what astronomers call a planetary nebula.
 | |||||||||
| The periodic table. The colors indicate some of the different nucleosynthetic sources for each element. (Credit: NAU Meteorite Laboratory). |
Stars take hydrogen and helium from BBN, and through various nuclear fusion processes, form other elements, namely, carbon, oxygen, silicon, sulfur, and iron. When these stars can no longer support themselves via fusion processes, their deaths release their processed material into their surrounding environment. The next generation of stars forms out of this enriched material and, upon its death, releases the complex elements it produced. Over time, the chemistry of the Universe gets increasingly complex and varied thanks to this cycle of stellar recycling. After BBN, approximately 99.9% of material was hydrogen and helium. Now, after over 10 billion years of recyling, approximately 2% of material is made of more complex elements. That may not seem like much, but consider that everything around you was produced during a star's life and death!

Comments
Post a Comment